Gerry Wright talks about the crisis of antibiotic resistance
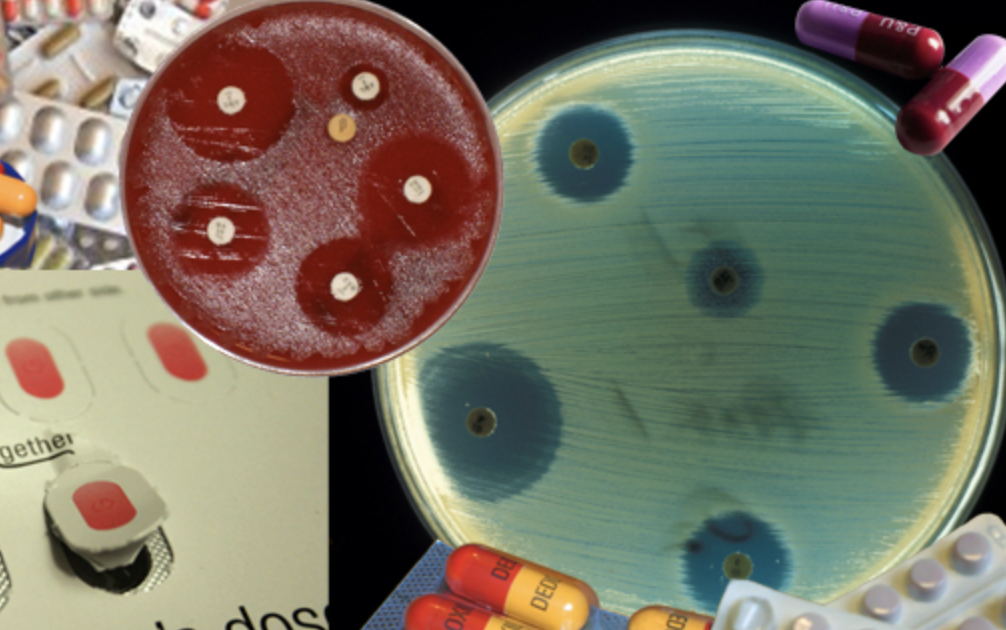
Antibiotics resistance refers to bacterial infections or fungal infections, not viruses. Through unnecessary use of antibiotics, the body’s bacteria can either mutate so antibiotic medicine is no longer effective or the bacteria acquires genes that confers resistance from other bacteria.
“It’s just a product of evolution. The selective pressure is very much an ‘adapt-or-die’ response,” said Gerard Wright, scientific director of the Michael G. DeGroote Institute for Infectious Disease Research and professor at McMaster University. “There’s a tremendous amount of pressure with the use of antibiotics on bacteria to either figure out a way around them or to simply wink out of existence.”
Resistance growing problem for nearly 70 years
This is not a new concern. Wright states this has been a concern since we started to use antibiotics. Sir Alexander Fleming, credited with the discovery of penicillin, an antibiotic substance, also foresaw danger in the 1940s. He warned that underdosage and unnecessary overexposure would make the body resistant to drugs when he accepted the Nobel Prize in Physiology or Medicine,
“It is not difficult to make microbes resistant to penicillin in the laboratory by exposing them to concentrations not sufficient to kill them, and the same thing has occasionally happened in the body. The time may come when penicillin can be bought by anyone in the shops. Then there is the danger that the ignorant man may easily underdose himself and by exposing his microbes to non-lethal quantities of the drug make them resistant.”
– Sir Alexander Fleming, Nobel lecture, December 1945
Which bacteria have developed antibiotic resistance?
According to Wright, it’s every bacterial infection.
“Every infection that has been treated with antibiotics is probably resistant. There’s no organism I’m aware of for which resistance has not become an issue,” said Wright. “The challenge right now is that for a good number of these, it’s not become resistant to just one antibiotic, but to many antibiotics. As we started using antibiotics in the early 40s to the present, if an organism developed a resistance to one, doctors just went up to the shelves and got another antibiotic of a different kind.”
The result is that bacteria is no longer resistant to the original antibiotic, but every single one since. Once a bacteria has the genes for resistance to one antibiotic, it keeps it. “So you have these situations now where one strain of bacteria is resistant to absolutely all of the antibiotics we have.”
We have bugs completely resistant; no ‘last resort’ antibiotics available
Two common strains of bacteria resistant to antibiotics today are MRSA (Methicillin-resistant Staphylococcus aureus) and CRE (carbapenem-resistant Enterobacteriaceae).
MRSA is an organism that causes skin infections, as well as pneumonia and blood infections. Methicillin refers to newer types of penicillin on the market. “But it’s not just resistant to that specific penicillin, but a whole pile of other antibiotics as well.”
Perhaps more concerning, and emerging in hospitals, are the CRE superbug.”It is resistant to the very last line of drugs we have.” This emerging superbug is a concern in hospitals. The deadly, untreatable infection leads to pneumonia, meningitis, wound infections, sepsis and many other infections
What can be done about antibiotic resistance?
Wright says doctors are good at making sure antibiotics aren’t prescribed for unnecessary circumstances, such as a cold.
“A cold is caused by a virus, viruses are not susceptible to antibiotics. You’re going to get better no matter what,” said Wright. “People should notpressure their physicians into giving them something. Listen to your doctor’s medcial advice and do not try to get extra drugs.”
This doesn’t mean avoid antibiotics altogether, however, cautions Wright. “Do not make that decision on your own. Seek medical attention. If you really need it though, of course, you should take an antibiotic or you could be potentially endangering yourself.”
Drug companies and governments need encouragement from the population to fund and research antibiotics.
“We need a whole bunch of new antibiotics which we don’t have right now,” said Wright. However, few pharmaceutical companies are looking into the area. Wright says there is a need for more research to figure out how to find new antibiotics.
“The traditional way we’ve done it in the past is not working anymore. We need to mix things up and challenge ourselves to try and think of new innovative ways to kill bacteria.”
Learning how to control bacteria infections has been instrumental in healthcare over the past 60 years. “Medical interventions like open-heart surgery, cancer chemotherapy, or hip and knee replacements. You can’t imagine doing those things without being able to control bacterial infection. If you don’t have antibiotics, you don’t have modern medicine.”
NewsRelated News
News Listing

McMaster Health Sciences ➚
IIDR member and founding director Gerry Wright among McMaster professors named to Canadian Academy of Health Sciences
News
September 10, 2024

McMaster Health Sciences ➚
IIDR’s Matthew Miller and Hendrik Poinar among Health Sciences faculty recognized by Royal Society of Canada
News
September 3, 2024
August 22, 2024

